The Chemistry of Nutrition and The Chemical Level of Organization

Atoms are the smallest units of matter that are stable. Atoms themselves are made up of positive protons, neutral neutrons (both in the center of the atom – the nucleus), and negative electrons in regular orbits about the nucleus.
Molecular bonding – Ionic bonds
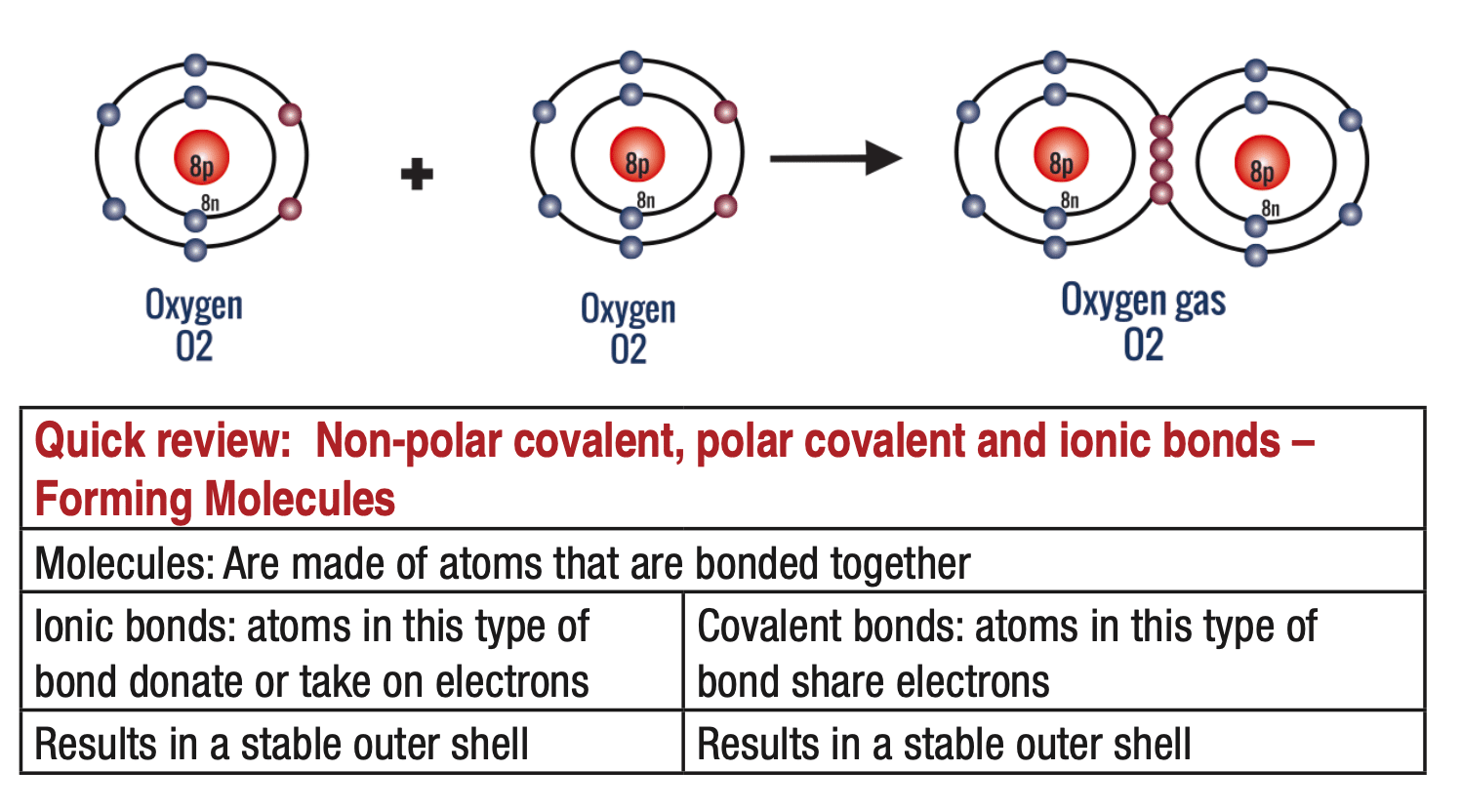
Ultimately, atoms are defined by the number of protons they have in the nucleus (the atom’s “Atomic Number”). Atoms will react with each other by either transferring or sharing electrons, with the ultimate goal of having a complete outer valence shell of electrons – this is the creation of molecules. It can be said that atoms are greedy or lazy when it comes to completing their outer shell of electrons. Some are greedy in that they will take electrons from a neighboring atom. In turn, the neighboring atom is lazy in that it would rather give up its outer shell electrons and drop down to the next full orbital. The atom which gains an electron will become a negative ion (anion) and the atom which loses an electron will become a positive ion (cation). In this case, an ionic bond is formed by the attraction between the two oppositely charged ions.
Molecular bonding – Covalent bonds
In other cases, both atoms are lazy, and rather than transferring electrons, it’s easier to simply share electrons in their outer shell. This is called a covalent bond. However, some atoms will have a greater attraction to electrons than others (electronegativity). When an atom in a covalent bond has a greater electronegativity than its neighbor, there will be an uneven sharing of valence electrons. The electrons would gather more around the more electronegative atom and that atom would have a partial negative charge. Likewise, the atom in the bond that has a smaller electronegativity will have a partially positive charge. This is known as a polar covalent bond and a great example of such a bond is in water, where the highly electronegative Oxygen will pull the electrons more around it. Therefore, Oxygen will have a partial negative charge, and the associated Hydro- gens will have a partial positive charge.
If the atoms have the same electronegativity (e.g. if the atoms are the same, such as the two oxygen atoms in O2) or nearly the same electronegativity (e.g. carbon and hydrogen have almost the same electronegativity, C-H), then the electrons would be shared evenly between the atoms, resulting in a non-polar covalent bond.
At this point, one may ask, “why is it important to understand these fundamental chemistry concepts in order to give nutrition recommendations to athletes?” Remember that nutrients are simply the molecules and ions that make up our cells. To truly comprehend how molecules such as carbohydrates, lipids, and proteins function, you must understand their unique structures and how their structures react in one more important molecule (and our other macronutrient), water.
Water and Hydrogen Bonds
With its unique properties, water is an incredibly important molecule for life. So that begs the question, what are the properties of water?
- Liquid at room temperature
- Liquid water does not change tempera-
- High heat of vaporization
- Frozen water is less dense than liquid water
- Molecules of water cling together
- A solvent for other polar molecules
Of course, this leads us to another question — Why does water have these unique properties? It is due to its shape, its polarity, and its creation of Hydrogen bonds (“1” on image to right).
Looking at Water
Water is formed by the covalent bond between an oxygen atom and 2 hydrogen atoms, therefore there is no “net” charge. However, oxygen has a greater electronegativity for electrons than hydrogen. Therefore, there is an unequal distribution of electrons, and a polar covalent bond is formed.
So how does this lead to the unique properties of water? What bond holds water molecules together? The creation of hydrogen bonds. Hydrogen bonds (H-bonds) occur between hydrogen in a covalent bond and a negatively charged atom of another molecule. These are relatively weak intermolecular (between molecules) bonds (approx. 5kcal/mol). Although relatively weak, the bonds are still bigger than room temperature (however, if one raises the temperature to boiling, the H-bonds will rip apart).
Hydrophobic vs Hydrophilic
Other polar molecules (e.g. carbohydrates) will dissolve in water, as the partial positive ends are attracted to the partially negative oxygen in water, and the partial negative end will be attracted to the hydrogen (hence, hydrogen bonds form between water molecules as well as between other molecules). Ions (e.g. Na+, Ca+2, Cl–) will also dissolve in water, with negative ions (anions) being sur- rounded by water’s partially positive hydrogen atoms and positive ions (cations) surrounded by the partially negative oxygens.
Nonpolar molecules (e.g. fats, oils, cholesterol) will not dissolve in water. This is the case for lipids, which are made primarily of C-C and C-H bonds. There are two reasons it will not dissolve in water. First, nonpolar molecules cannot form H-bonds with H20. Second, water would have to order itself around each molecule in what is known as a loss of entropy (chaos or randomness). This organization would require energy.
From this point forward, we can classify molecules not only as polar and nonpolar but also if they will dissolve in water. Lipids are hydrophobic (hydro– mean- ing water, and –phobic meaning fear) molecules and will not dissolve in water while carbohydrates and proteins are hydrophilic (-philic meaning love) that CAN form H-bonds and dissolve in H2O.
Amphipathic molecules, Polar and Nonpolar at the same time
Some very specific (in our in case, very important) molecules are hydrophilic/polar at one end and hydrophobic/ nonpolar at the other. These are known as amphipathic molecules. If put in water, the hydrophilic end of these molecules will point out in the water and the hydrophobic end will associate with other hydrophobic ends. In this case, a micelle is created.

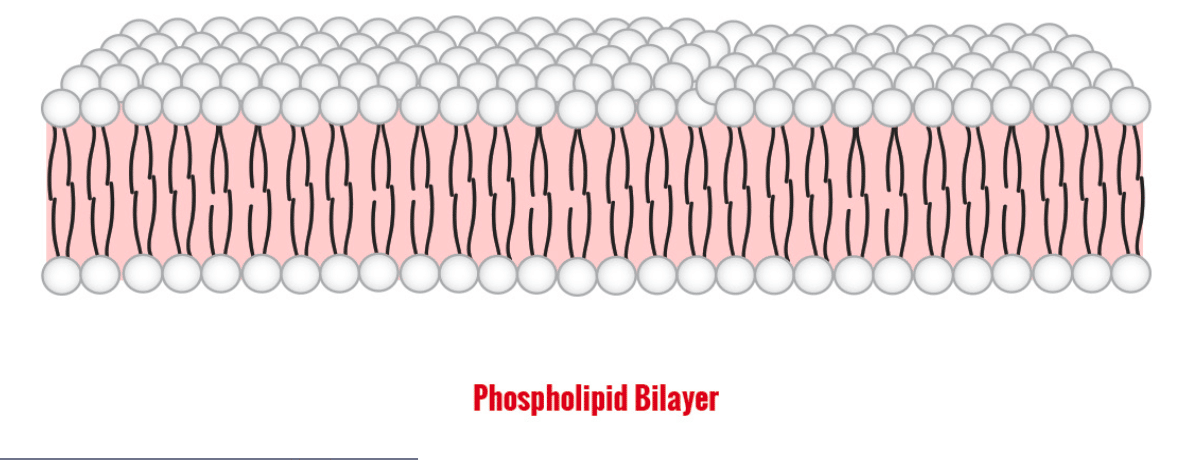
The Lipid Bilayer of Biological Membranes
More importantly for our purposes, amphipathic molecules known as phospholipids are the primary constituent of all cell membranes. Phospholipids are very similar in structure to triglycerides, the main type of fat in your body (its structure will be discussed later). Both phospholipids and triglycerides have a 3-carbon glycerol backbone, but while triglycerides have three fatty acids attached to each of the carbons in glycerol, phospholipids are a bit different. Phospholipids have only two hydrophobic fatty acid “tails” and a hydrophilic “head” consisting of a phosphate group. All cell membranes, including the outer plasma membrane, consist of two layers of phospholipids with the hydrophilic phosphate groups on the outside of the layer and the hydrophobic lipid tails in the center. The important thing to realize is that these cellular membranes separate two aqueous spaces (e.g. the inside/intracellular and the outside/extracellular).
Important Chemical Reactions of the Cell
Making and Breaking Organic Molecules: Dehydration-Hydrolysis Reactions
The large macromolecules of primary concern in nutrition science, large (complex) carbohydrates, proteins, and lipids are made of smaller components. Both complex carbohydrates and proteins are considered polymers (poly– meaning “many”), meaning they are made up of individual subunits, monomers (mono– meaning “one”). The monomers of complex carbohydrates are known as monosaccharides, while the monomers of proteins are amino acids. Lipids are a bit different as triglycerides (the category of lipid that includes fats and oils) are made not of monomers, but as noted above, consist of three fatty acid chains connected to glycerol. These large macromolecules are continually being broken down to their original subunits (e.g. monomers). Likewise, monomers are continually being combined into their larger forms (macromolecules).

The synthesis of macromolecules is via a specific class of chemical reactions known as dehydration reactions. In dehydration reactions, it is the removal of water that allows subunits to link together into larger molecules (hence the term “dehydration”, de– meaning removing, –hydration meaning water). A common dehydration reaction that we will see is known as “Esterification,” which is what happens when a fatty acid reacts with glycerol to form triglycerides.
The reverse reaction, breaking down larger organic molecules into smaller ones is known as hydrolysis. In a hydrolysis reaction, it is the addition of water that breaks larger molecules into their subunits. Again, the naming convention makes sense (hydro– meaning water, –lysis meaning to unbind).
Introduction to Macromolecules
Part 1: Simple and Complex Carbohydrates
Carbohydrates are a class of molecules made of Carbon, Hydrogen, and Oxygen in which the Hydrogen and Oxygen atoms are in a 2:1 ratio. Hence, the empirical formula for all individual carbohydrates is Cn(H2O)n. The individual subunits (monomers) of carbohydrates are called monosaccharides.
- ♦ Example: Glucose (6 C atoms) => Hexose
- ♦ Example: Glycerol (3 C atoms) => Triose
NOTE: suffix “______ose” used for carbohydrates (as opposed to “______ase” for enzymes)
The primary function of carbohydrates is as short and long-term energy storage. Carbohydrates are found in “simple” and “complex” forms.
Simple Carbohydrates are either one carbohydrate monomer (monosaccharide) or two monosaccharides bonded together by a dehydration reaction (disaccharide).
Monosaccharides – 1 carbon ring (ex: Glucose, Fructose, and Galactose)
- Glucose – C6H12O6
- Fructose – C6H12O6
- Galactose – C6H12O6
Glucose, galactose, and fructose are all hexoses. They are structural isomers, meaning they have the same chemical formula (C6H12O6) but a different arrangement of
atoms giving them different biochemical characteristics.
Glucose (a.k.a. dextrose or blood sugar) is the most important monosaccharide in the human body. It is used directly by the cell for energy. It is stored as glycogen in the muscles and liver for later use (please see below). It can also be converted to fat when glycogen stores are full and stored for energy.
Fructose is also known as fruit sugar. The liver converts fructose to glucose for usage by the cells. Galactose forms milk sugar or lactose. Again, the body must convert galactose to glucose for energy metabolism.
Disaccharide – 2 carbon rings (ex: Maltose, Sucrose, and Lactose).
Note, that each disaccharide includes glucose as a principal component.
Sucrose is the most common dietary disaccharide and occurs naturally in most foods that contain carbohydrates, refined to make table sugar.
Lactose is found in natural form only in milk.
Maltose occurs in beer, cereals, and germinating seeds, also known as malt sugar
Polymerization of Carbohydrates – Making Polysaccharides
Recall that macromolecules can be created via polymerization (as a polymer) made up of individual subunit monomers. In the case of Carbohydrates, notice that the side hydroxyl (OH) groups on monosaccharides are ALL potential sites for dehydration reactions to create complex carbohydrates (polysaccharides – carbohydrate polymers). The bonds linking monosaccharides are known as glycosidic bonds.
Polysaccharides are typically differentiated by their source – plant or animal.
Glycogen is the storage form in animals (cross-branched like a Christmas tree). Stores glucose in a metabolically inactive state.
Starch and fiber are two common forms of plant polysaccharides.
In an average adult, approximately 1400 kcals of glycogen are stored in the muscle and 320 kcals in the liver.
Plant starch accounts for approximately 50% of the total carbohydrate intake of Americans and a major source of calories. Fiber, on the other hand, resist hydrolysis in the digestive tract. While fiber does not contribute as much to caloric intake as starch, there are many health benefits. Fiber retains water and therefore gives “bulk” to the food residues in the instestines, making one feel full. It also exerts a scraping action on the inner lining of the digestive tract. In a sense, it helps “clean the GI tract.” Fiber also binds or dilutes harmful chemicals as well as shortening the transit time for food residues (and possibly carcinogenic materials) to pass through the digestive tract.
How Can You Learn More?
The NESTA Sports Nutrition Specialist course is designed for personal fitness trainers, strength coaches and nutrition experts who want to learn cutting-edge techniques for increasing sports performance, reducing recovery time, and enhancing the overall well-being of your clients and athletes.
If you want to help clients with food, diet, weight management and improving the results of their fitness routines, the Fitness Nutrition Coach course is for you. You will learn about optimal nutrition, including proven techniques for increasing energy, optimal health and decreased dependence on medications. Instantly increase your job and career opportunities with this popular professional credential.
You can become a Certified Personal Fitness Chef and expand your current personal chef business, or add a new profit center for your fitness or wellness business. Many personal chefs cook and coach people in groups to help more people and earn more money per hour. Some chefs provide weekly meal prep service for health-minded customers and athletes.
Check out what it takes to start a career in personal fitness training. This is your most affordable and fastest way to become a highly qualified personal trainer.
NESTA coaching programs are open to anyone with a desire to learn and help others. There are no prerequisites.
That’s it for now.
Take action!
PS: Click here to see many helpful business/career resources