What are Epigenics and How Does It Play a Roll in Aging?
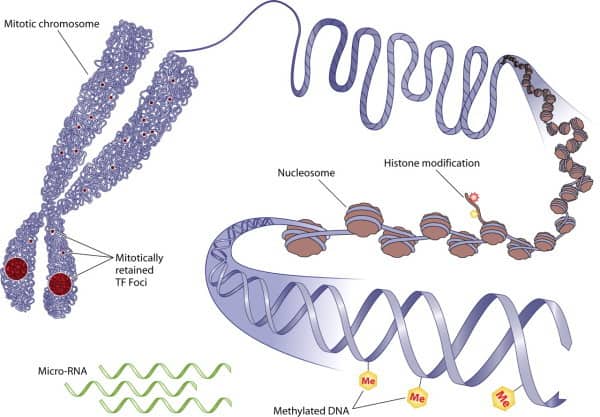
Epigenetics is a term used for gene expression modifications caused by heritable, but potentially reversible, changes in DNA linked processes. These processes include DNA methylations, modifications of histones and chromatin structural changes.
DNA methylation modifies a DNA strand after replication. It is the addition of a CH3 (methyl group) to a nucleoside residue – most often cytosine. DNA methylation is an important factor in normal development and in cellular differentiation and gene expression. There are many ways that gene expression is controlled and methylation of DNA is but one – but a very important one. It is the process that metaphorically turns genes “off”. Thus, methylation is seen as reversible and the extent and timing of turning the gene “on” is critical to growth, development and often, health and disease. DNA methylation dysfunction is seen in virtually all cancers and is implicated in many chronic disease states associated with aging such as diabetes and cardiovascular disease.
Histones are proteins that condense DNA in the nucleus of cells and they play an important role in gene regulation by compacting DNA. The DNA/histones complex, and its functionality, allows for controlled access to the DNA strands by gene modulating and reaction initiating molecules. DNA, when in a histone complex, is actually not a linear strand but rather it is intertwined with the histone proteins so it may fit into the nucleus. The combination of DNA and histone proteins form what is called chromatin. Chromatin appear like beads on a string. The beads are called the nucleosomes and each nucleosome is the DNA wrapped around the histones. Thus, we have this rather complex and elegant structure of the chromosome that protects and plays an important role in how the genetic material manifests its characteristics.
It may be intuitive from previous posts that due to the fact that the glycation reaction targets proteins and nucleic acid residue amine functionality the nuclear material is in harms way with relation to glycation reactions. And, in fact, there is significant research that supports this.
In other posts we have seen that the metabolism of carbohydrates can produce a strong glycating reactant, methylglyoxal (MG). It has been reported (1) that MG reacts with histone proteins to create structural modifications. The modifications show various conformational changes that can lead to lack of chromatin integrity and result in various pathological conditions resulting from DNA disorder.
Histone H1 plays an important role in the packing of chromatin and controlling gene expression. Under conditions of hyperglycemia, as typically found in uncontrolled diabetes for example, the reaction of nonenzymatic glycation on the structure of histone H1 (2) was shown to alter H1 folding and reduced DNA/H1 binding. In this study, the changes in the structure and function of histone H1 is suggested as one of the possible mechanisms likely involved in diabetic complications.
It is apparent, and understandable, that glycation can easily disrupt the structure of histones and chromatin and potentially lead to a myriad of scenarios of chromatin/histone dysfunction. This type of dysfunction is seen in a variety of diseases and in the aging phenotype. The role of glycation in DNA methylation, however, is not as direct.
In mammals, cells are genetically identical but via processes such as DNA methylation, genes are in active or inactive (“on” or “off” as we have said) mode and when required they are induced to drive cellular differentiation into specific cell types. Chromatin’s intact structure is required to maintain integrity of this process. The complexity of replication and differentiation process is regulated by epigenetic mechanisms, which are associated with the DNA, histones, and nucleosomes in the cellular nuclei. In an excellent review by Sadakierska-Chudy (3), DNA methylation, cytosine derivatives, active and passive demethylation pathways, as well as histone variants, were examined as was the role that these processes play in physiological integrity. Histone variations within the nucleosomes create structural and functional controls within chromatin. Any compromised structural aspects of histones can compromise accessibility control of DNA and potentially factor in the methylation and demethylation processes. This in turn will affect multiple biological processes, such as replication, transcription, DNA repair, and play a role in various disorders including cancer. And as we have indicated, we have seen one of the ways this histone dysregulation is created is by histone protein glycation. So again we can see the role of glycation in another of the hallmarks of aging
Next:
Hallmarks of Aging
Role of Glycation Part 5: Loss of Proteostasis
- Mir, AR, et al, Methylglyoxal mediated conformational changes in histone H2A – generation of carboxyethylated advanced glycation end products. 2014, Int J Biol 260-6
- Rahmanpour R, et al, Histone H1 structural changes and its interaction with DNA in the presence of high glucose concentration in vivo and in vitro. 2011, J Biomol Struct Dyn.
- Sadakierska-Chudy, A., et al, A Comprehensive View of the
Epigenetic Landscape Part I: DNA Methylation, Passive and Active DNA Demethylation Pathways and Histone Variants, 2014, Neurotox Res.
Nick Pokoluk is a biochemist, Six Sigma Black Belt of Transformational Change Methodology and certified wellness coach. He can be reached at npokoluk@wwtpi.com or openlcr@yahoo.com